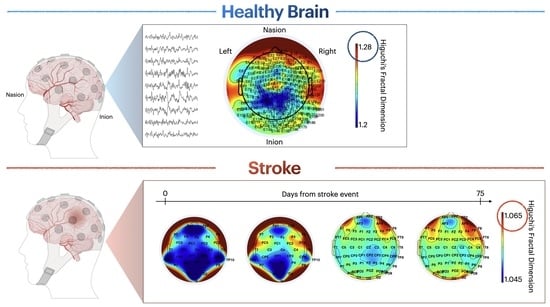
Appendix A. FD Measures
The FD provides a complexity index that describes how the measure of the length of a curve changes depending on the scale used as a unit of measurement. Different approaches can be used to estimate FD [
32]. Among these approximation techniques, the Higuchi FD standard approach calculates the FD of a time series in the time domain and is based on the
versus
curve (referred to
in the paper) computed as follows. (a) For each sample
i of the EEG epoch
of length
N, the absolute differences between values
and
—i.e., the samples at distance
k—are computed, considering
; (b) then, each absolute difference is multiplied by a normalization coefficient that takes into account the different numbers of samples available for each value of
k. The computation of this coefficient is based on the starting point
and on the total number
N of samples of an epoch; (c)
is computed as:
where
and
is computed by summing the obtained values and dividing by
k.
By definition, if
is proportional to
for
where
defines the upper range of the linear region of
, then the curve is fractal with dimension
D. Thus, the Higuchi FD increases as the signal irregularity increases [
16,
17]. We considered a wider domain of
with respect to the Higuchi FD [
16]: we extracted two additional features that considered the oscillatory behaviour of the nonlinear part of
(
) whose features depend on the periodicity of the signal itself [
16]. The FD Residuals evaluates the deviation—i.e., the sum of the squares of the residuals—of the curve from the regression line computed in its linear region (
). The second feature is the FD Tortuosity,
. This measures the rate at which the curve changes with respect to its coordinates changes (
and
) by using their first and second partial differences:
where
,
,
, and
. These two features increase as the signal periodicity increases [
17]. FD Residuals and Tortuosity are estimated extending their quantification to the nonlinear part of the curve
. When
k is comparable to the number of samples comprised in one oscillation of the original signal—i.e., a period—it will result in a low value of
—i.e., a negative spike increasing the value of both FD Residuals and Tortuosity, indeed the greater the number of changes in the curvature sign, the more tortuous the signal. We refer the reader to [
16,
33] for further details on these FD measures extraction.
For the EEG FD measures, the linear region was defined by imposing a k
lin equal to 6, according to [
34], while for the Residuals and the Tortuosity, k
max was set as equal to 100, k
max ≥ k
lin according to [
16].
In
Figure A1, we reported a toy example to illustrate how FD Higuchi, Tortuosity and Residuals are computed from the
versus
curve estimated from a time-series.
In the Results section, for the sake of simplicity, we only reported the results referred to FD Higuchi and Tortuosity. In
Figure A2, we reported the results of FD Residuals that give us a quantification of how much the interpolation line of the linear region of the fractal curve differs from the nonlinear part of the fractal curve. On average, controls have lower FD Residuals values (i.e., range [165, 195]) and their distribution mainly differ in frontal areas compared to stroke survivors ([200, 350]).
In
Figure A3, we reported the results of all the FD measures and clinical scales for stroke survivors as box-plots.
Figure A1.
Example of versus curve (referred to in the paper) computed from a cosine with 7.5 Hz of frequency. The slope of the linear part of the curve stands for the FD Higuchi (in green), the changes in the curvature sign for values greater than (in red) stands for the FD Tortuosity (in light blue) and the deviation of the curve from the regression line computed in its linear region stands for the FD Residuals (in violet).
Figure A1.
Example of versus curve (referred to in the paper) computed from a cosine with 7.5 Hz of frequency. The slope of the linear part of the curve stands for the FD Higuchi (in green), the changes in the curvature sign for values greater than (in red) stands for the FD Tortuosity (in light blue) and the deviation of the curve from the regression line computed in its linear region stands for the FD Residuals (in violet).
Figure A2.
Topographic maps of the median values of FD Residuals in healthy controls (first row, colormap range is from 165 to 195) and during acute (second and third row) and sub-acute (fourth and fifth row) phase in stroke survivors (colormaps range is from 200 to 350). Second and fourth rows refer to stroke survivors with right-hemisphere lesion, third and fifth rows to stroke survivors with left-hemisphere lesion. Second and fourth columns report Wilcoxon test statistic (stroke survivors vs. controls, colormaps range is from 0 to 0.016 for Dataset 1, i.e., Acute Phase, and from 0 to for Dataset 2, i.e., Subacute Phase).
Figure A2.
Topographic maps of the median values of FD Residuals in healthy controls (first row, colormap range is from 165 to 195) and during acute (second and third row) and sub-acute (fourth and fifth row) phase in stroke survivors (colormaps range is from 200 to 350). Second and fourth rows refer to stroke survivors with right-hemisphere lesion, third and fifth rows to stroke survivors with left-hemisphere lesion. Second and fourth columns report Wilcoxon test statistic (stroke survivors vs. controls, colormaps range is from 0 to 0.016 for Dataset 1, i.e., Acute Phase, and from 0 to for Dataset 2, i.e., Subacute Phase).
Figure A3.
Results of averaged FD measures—respectively of Higuchi, Residuals and Tortuosity—at T0 (in green) and T1 (in violet) in acute phase (first row) and between (in violet) and T2 (in yellow) in subacute phase (third row). Clinical recovery was estimated through NHISS and FMA scores at T0 and T1 (in acute phase—second row) and through ARAT, BBT and N-HPT scores at and T2 (in sub-acute phase—fourth row). Results for participants with right hemisphere lesion (first two columns in each plot) and participants with left hemisphere lesion (last two columns in each plot) are reported on separate columns for the sake of clarity. On each box, the central line indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers, and the outliers are plotted individually using the ‘o’ symbol.
Figure A3.
Results of averaged FD measures—respectively of Higuchi, Residuals and Tortuosity—at T0 (in green) and T1 (in violet) in acute phase (first row) and between (in violet) and T2 (in yellow) in subacute phase (third row). Clinical recovery was estimated through NHISS and FMA scores at T0 and T1 (in acute phase—second row) and through ARAT, BBT and N-HPT scores at and T2 (in sub-acute phase—fourth row). Results for participants with right hemisphere lesion (first two columns in each plot) and participants with left hemisphere lesion (last two columns in each plot) are reported on separate columns for the sake of clarity. On each box, the central line indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers, and the outliers are plotted individually using the ‘o’ symbol.
Appendix B. Supplementary Statistical Comparisons
For sake of completeness, we compared the FD features among the same participants between T0 and T1 (for Dataset 1) and between
and T2 (for Dataset 2) (
Figure A4). Because FD measures were not normally distributed, non-parametric tests were applied: a paired sample two-sided Wilcoxon signed rank test was performed to identify significant differences at topographical level between FD features at T0, T1,
and T2.
We can observe that FD Higuchi gradually increases already from the early hours after the event in stroke survivors (
Figure A4—first and second row). Interestingly, this phenomenon occurred exclusively in those with a right (non-dominant) hemispheric lesion with a
(
Figure A4—first and third row).
Figure A4.
Results in acute phase (first and second row, colormap range from 1.042 to 1.058) and sub-acute phase (third and fourth row, colormap range from 1.05 and 1.065) for Fractal Dimension Higuchi. Topographic map of: (1) the median values of the Fractal Dimension Higuchi among survivors with right hemisphere (non-dominant) lesion and left hemisphere (dominant) lesion (first and second column) and (2) Wilcoxon test statistic (T0–T1 in acute phase and –T2 in subacute phase) where crosses stand for significant increase ( < 0.05; colormap range from 0 to 1) (third column).
Figure A4.
Results in acute phase (first and second row, colormap range from 1.042 to 1.058) and sub-acute phase (third and fourth row, colormap range from 1.05 and 1.065) for Fractal Dimension Higuchi. Topographic map of: (1) the median values of the Fractal Dimension Higuchi among survivors with right hemisphere (non-dominant) lesion and left hemisphere (dominant) lesion (first and second column) and (2) Wilcoxon test statistic (T0–T1 in acute phase and –T2 in subacute phase) where crosses stand for significant increase ( < 0.05; colormap range from 0 to 1) (third column).
We also compared the FD features among Dataset 1 at T1 and Dataset 2 at
. We reported in
Figure A5, the t-maps of the FD features that resulted significantly different (
< 0.05): interestingly there was a gradually decrease comparing T1 in acute phase vs.
in the sub-acute phase only in participant with left-hemisphere lesion.
Figure A5.
t-maps representing Wilcoxon test statistic (T1 in acute phase vs. in subacute phase) where non-white colors stand for significant difference ( < 0.05; colormap range from 0 to 0.05). A significant decrease between T1 in acute phase vs. in subacute phase was observed only for participants with left-hemisphere lesion.
Figure A5.
t-maps representing Wilcoxon test statistic (T1 in acute phase vs. in subacute phase) where non-white colors stand for significant difference ( < 0.05; colormap range from 0 to 0.05). A significant decrease between T1 in acute phase vs. in subacute phase was observed only for participants with left-hemisphere lesion.